Clinical Trials
Clinical Trial Management System (CTMS)
Please ensure that you have explored the new CTMS system. From 01 September 2023, NSW Health is mandating the use of the Clinical Trial Management System (CTMS) for all new clinical trials conducted across New South Wales.
All SESLHD clinical trial governance applications must include:
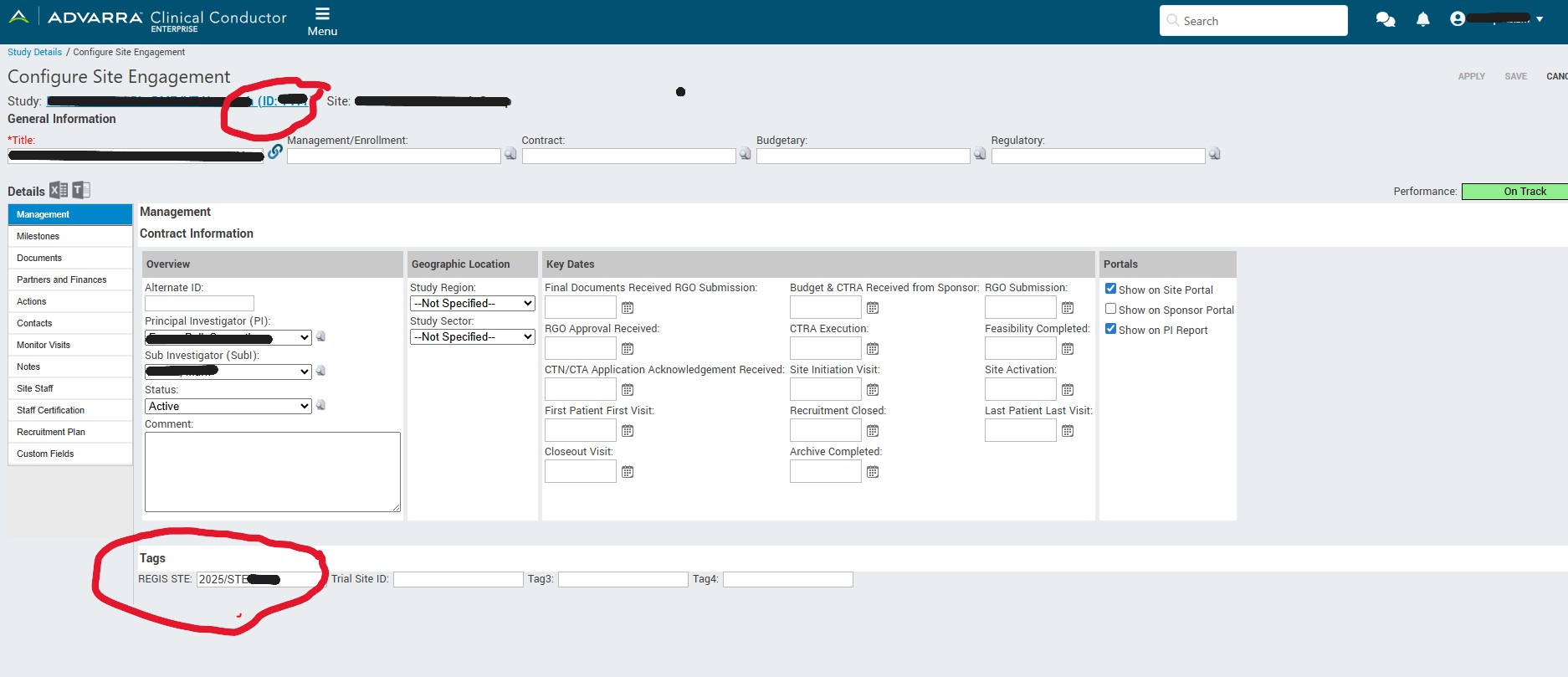
- A screenshot of the study Minimum Data Set entered into the CTMS (see example below)
- The study CCID code
Clinical trials will not receive full governance authorisation until the study has been entered into the CTMS.
For CTMS training, resources and user accounts, please register here.
Clinical Trials Governance Framework Implementation Working Group
The Australian Commission on Safety and Quality in Health Care (ACSQHC), on behalf of all jurisdictions, developed the National Clinical Trials Governance Framework (Governance Framework) as the first step towards a nationally consistent approach to the accreditation of health services for the conduct of clinical trials. The Framework will ensure health service organisations comply with the actions in the National Safety and Quality Health Service (NSQHS) Standards 1 and 2 for clinical trial services as provided in the Framework, and to identify additional resources that may be required for SESLHD to meet implementation requirements.
Clinical trial governance framework working party meetings are being organised. If you would like to be part of the meetings – please email: SESLHD-RSO@health.nsw.gov.au
For clinical trial governance work plans:
- SESLHD Clinical Trial Governance Framework workplan template– standard 1
- SESLHD Clinical Trial Governance Framework workplan template – standard 2
Please email SESLHD-RSO@health.nsw.gov.au for further information or to be included in the working group for SESLHD implementation of the Clinical Trials Governance Framework.
Clinical Trials Register of Staff
The Office for Health and Medical Research has established the Clinical Trials Register of Staff (CiTRoS) to give all contributors and NSW Health, a better understanding of Australia’s dynamic clinical trials workforce.
All data entered will complement the National Clinical Trial Governance Framework requirements and be a reference for future local, state and national initiatives to enhance the capability and capacity of the clinical trials workforce.
How it helps you
If you play a role in clinical trials such as clinical trials unit staff, research office staff, investigators or supporting department staff, you and your team will benefit from adding data into CiTRoS. CiTRoS will capture the knowledge and experience you bring to your clinical trials role, the position you are currently working in, the educational opportunities you seek, and the career progression pathways you need.
CiTRoS is secure
- Once your data has been entered into the CiTRoS database, it can be accessed and updated by you at any time.
- Identifiable information is required for a personalised link to access your database record and to initiate any requested mailing lists or communication strategies.
- Any reports generated for outside your organisation, will only be provided as unidentifiable collated data.
Be part of this exciting initiative by submitting your CiTRoS record here.
Help us to understand you better, to identify any gaps, and to work through improving our workforce together. It should only take about 5-10 minutes to create your own CiTRoS record. Please also share this opportunity with any colleagues working in research.
You can find more information on the CiTRoS website or by contacting clinicaltrialsnsw@health.nsw.gov.au