Site Specific Authorisation
Site Specific Assessment (SSA) applications will be reviewed by the Research Ethics and Governance Office in accordance with NSW Health PD 2010_056 Research - Authorisation to Commence Human Research in NSW Public Health Organisations.
Please note: You cannot commence your study until you have SSA authorisation.
Please ensure before submitting your SSA:
- That you have referred to the presubmission guide
- That you have personally contacted the Head/s of Department/s (HoD) to discuss your resource request, ensuring they are the correct HOD and have had the opportunity to ask questions.
- That you have the correct CPI and PI listed within your SSA. Please refer to the OHMR website for clarification of roles.
- That you have successfully gained Contingency Worker Status for each research team member who are not SESLHD employees. For information on contingent worker onboarding and access to SESLHD sites, please contact SESLHD-PositionMaintenance@health.nsw.gov.au
- That you have completed and complied with the Governance Checklist or it will be ineligible for review.
- That you agree to return requested information from the Research Office within 30 days of the request, or your project will be withdrawn.
For Governance Site-Specific Applications (SSA), the following must be included in the submission to REGIS along with other project-specific documents for governance review to commence:
- Method of Payment Form
- SESLHD Research Project Budget Template
- Research Governance Submission Checklist for Researchers
Site-Specific Authorisation (SSA) Timeline
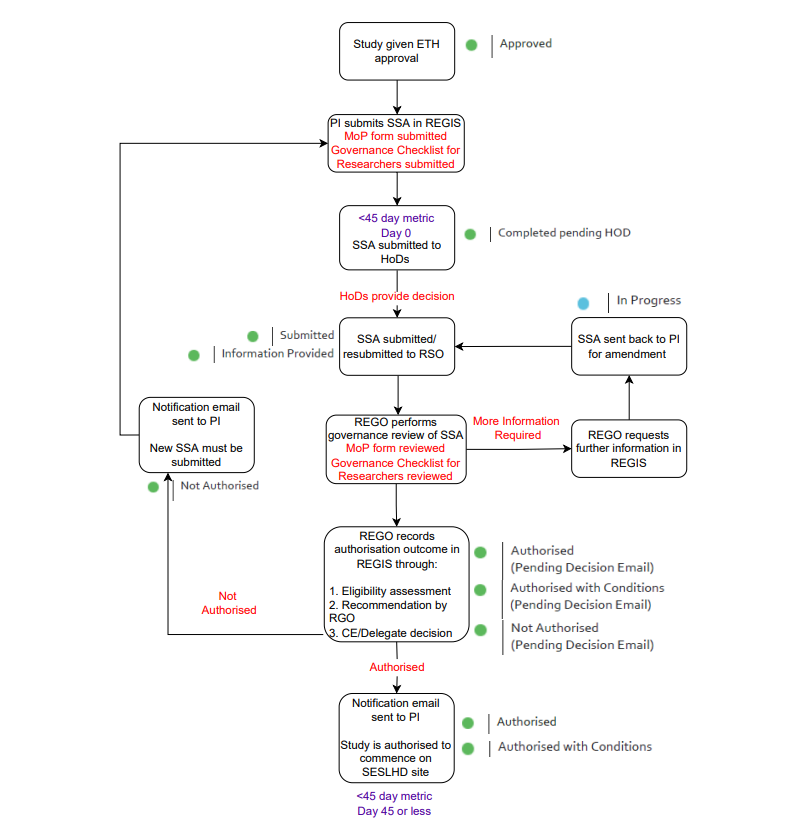
Requirements for SSA Applications
All non SESLHD staff team members will require Contingency Worker Status for the study to gain Site Specific Authorisation and commence the study. Please contact SESLHD People and Culture on SESLHD-PositionMaintenance@health.nsw.gov.au
To AVOID DELAYS - PLEASE ENSURE YOU HAVE CWS AND ALL OTHER RELEVANT DOCUMENTS BEFORE SUBMITTING YOUR GOVERNANCE APPLICATION
Research project budgets must be itemised using either the SESLHD Research Project Budget Template or the OHMR Clinical Trial Budget Costing Tool.
The budget must be attached to your SSA and approved by the Head of Department (HoD) prior to being submitted to the Research Office.
The resource requirements for conducting research must be approved by the Head of Department (HoD) in which the study is taking place, as well as HODs that will support the study.
Examples of supporting departments include:
- Pharmacy - Studies involving the use of drugs must be approved by the on-site pharmacy.
- Radiology - Studies involving imaging must be approved by the on-site radiology department.
- Health Information Unit - Studies requiring access to archived medical records must be approved by the site HIU.
- NSW Health Pathology - For studies requiring support from NSWHP for venepuncture, sample processing, pathology testing or access to tissues held by anatomical pathology, an order form must be submitted to NSWHP. The returned quote will be accepted as an indication of NSWHPs ability to support the study.
In the event that the HoD is on the study team, the resource requirements will need to be approved by their line manager.
Correspondence from a Lead HREC certified by the National Mutual Acceptance Scheme approving conduct of the study at the site is required. For studies within SESLHD, the accredited lead is the SESLHD HREC.
When a project is approved by a Lead HREC as a multi-centre project, study master documentation will need to be customised for each site. Documents for use at SESLHD sites will need to:
- Display the SESLHD or facility logo
- Include details of the site contact person
- Direct complaints to the SESLHD Research Ethics and Governance Office: Ph: (02) 8797 7605 - Please leave a message with your contact details | E: SESLHD-RSO@health.nsw.gov.au
Research that exposes humans to ionising radiation must be submitted to the site Radiation Safety Officer. The Radiation Safety Report must be attached to the SSA.
Contracts and indemnities are to be submitted via REGIS as part of the SSA and must be signed by the Sponsor and PI prior to submission. The Research Office accepts scanned and electronic signatures and will return scanned signatures back to research teams via REGIS. Please note that the Research Office is paperless and cannot return original documents.
Details to be used on contracts are:
| Business name | South Eastern Sydney Local Health District |
| Address |
District Executive Unit, Level 4 The Sutherland Hospital & Community Health Service Cnr The Kingsway and Kareena Road CARINGBAH NSW 2229 |
| ABN | 70 442 041 439 |
| Contact for notices | Site Principal Investigator |
The standard agreements available on Medicines Australia, without alteration, are accepted. The process for requesting changes to these agreements is managed outside of SESLHD. Further information is available on the OHMR website.
Further information about different agreements is as follows:
- Material Transfer Agreements (MTAs) - MTAs are required where HUMAN TISSUE and/or DATA is being provided to an external institute for research purposes. As a standard agreement is not yet available, please contact the Research Office for guidance.
- Collaborative Research Group Agreements - Agreements need to be in place between collaborating institutions to ensure that arrangements are in place for funding, intellectual property ownership, indemnity, liability etc. Agreements may only be submitted to REGIS once the collaborative group and the Site Principal Investigator have signed the agreement.
- Clinical Trial Research Agreements (CTRA) - All commercially sponsored clinical trials require a CTRA. For commercially sponsored studies of medical technologies and devices, the standard agreement developed by the Medical Technology Association of Australia will be accepted.
- Indemnity - Sponsors of commercial trials must provide indemnity to SESLHD. Available here.
Commercially sponsored and collaborative group trials require a current insurance certificate held in the name of the local sponsor with AUS $20M cover.
Students and external researchers must provide evidence of insurance cover from their university or employer, as stated under the Site Team section.
Clinical trial sponsors are required to notify or seek exemption from the TGA when they intend to use unapproved therapeutic goods. Evidence of CTN or CTX submission will need to be attached to the SSA.
Where SESLHD is the sponsor, the Research Office will submit the CTN or CTX in conjunction with the Principal Investigator. In this instance, please contact the office prior to submitting your SSA.