Ethics Application Process
Please see the Ethics Submission Checklist for guidance on submitting your application.
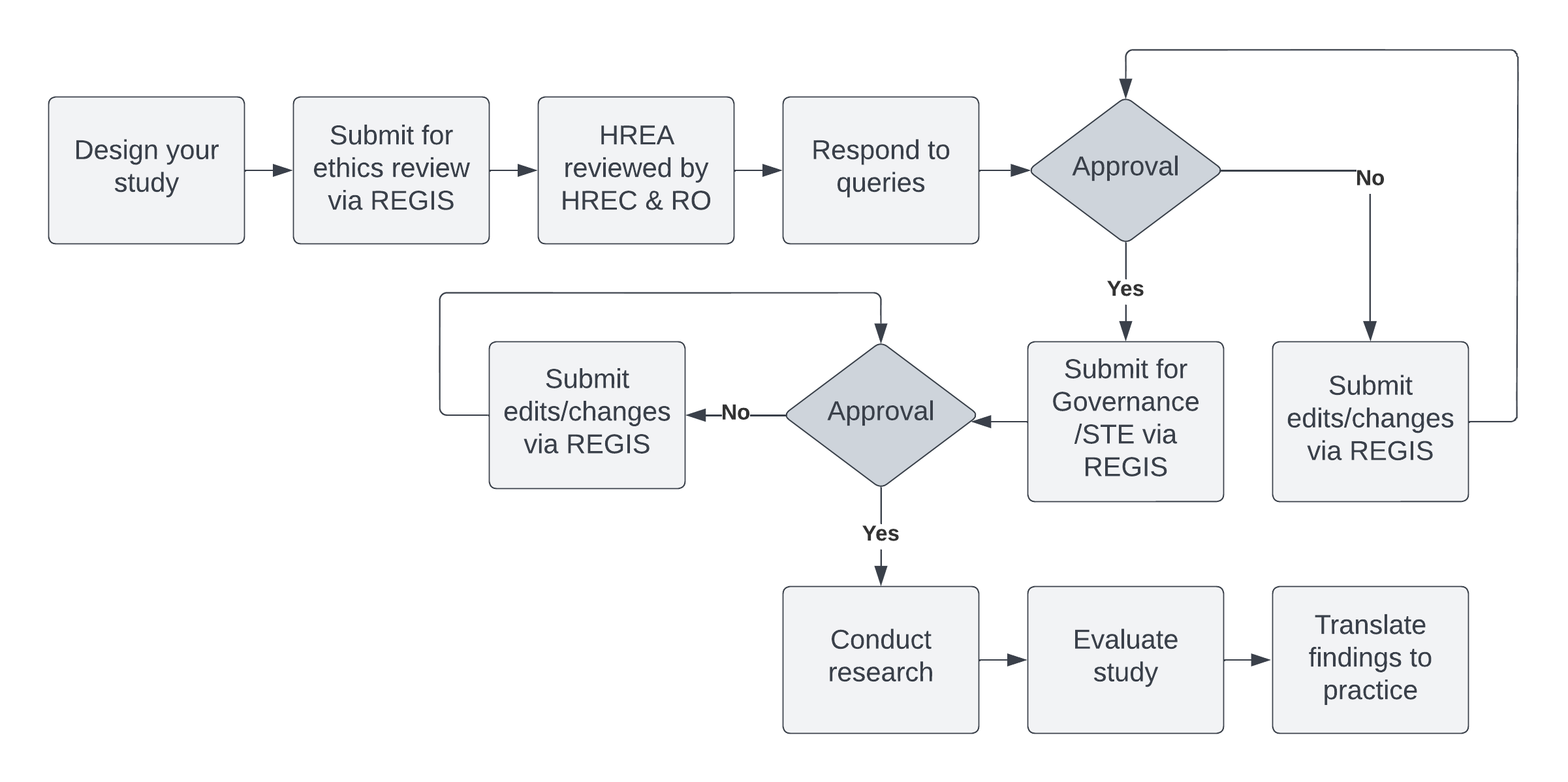
Submissions must be made via REGIS. In your application you will be required to nominate a review pathway. This is determined by a project's level of risk (i.e. Low Risk or Greater than Low Risk). If you are uncertain of which pathway your research falls under, contact the Research Office. Low Risk Applications are reviewed via the Low Risk Ethics Committee.
SESLHD Human Research Ethics Committee (HREC)
The SESLHD Human Research Ethics Committee (HREC) is a registered and certified HREC which can provide ethical review for single-centre and multi-centre research projects as part the National Mutual Acceptance initiative in the following areas of research: Clinical trials – Phase II, III, IV, Clinical interventional research other than clinical trials, Mental health, Paediatrics, Population health and/or public health and Qualitative health research.
Please see HREC meeting and submission dates to avoid delay in project review and approval.
What is an Human Research Ethics Application (HREA)?
A Human Research Ethics Application (HREA) is a web-based form developed by the National Health and Medical Research Council (NHMRC) to assist researchers consider and address the ethical principles set out by the National Statement on Ethical Conduct in Human Research (2007). In NSW, HREA are completed by the REGIS platform. Quick Reference Guides are available to assist researchers in preparing their HREA and submitting through the REGIS.
Please note, when preparing an HREA it is important you provide all relevant details and documentation. This includes ensuring details are accurate and consistent across ALL documentation you have provided. For example, if you propose to conduct a study over multiple sites, you will need to list each site on the project registration, protocol and HREA. Similarly, you will need to ensure all the names and roles of the research team are consistent across documentation too.
Responses to Committee queries
When responding to queries please follow the instructions in your request email and provide a cover letter which addresses (point-wise) the questions raised by the Committee as well as tracked and clean copies of amended documents. Please ensure version numbers and dates are updated.
Quality Improvement Activity and Ethical Review
NSW Health adopts the distinctions between projects requiring ethical review and those that do not as set out by the NSW Health Guideline: GL2007_020 - Human Research Ethics Committees - Quality Improvement & Ethical Review: A Practice Guide for NSW.
Researchers conducting human research are required to submit their projects to a Human Research Ethics Committee as per NSW Health Policy.
NSW Health encourages the widespread implementation of Quality Assurance (QA) programs as an essential part of a learning healthcare organisation. QA should be conducted as part of conventional clinical service delivery or 'business-as-usual'. In most routine situations, QA projects should not require ethical review.
QA practitioners should complete a QA Screening Form (link below) which will assist in assessing the characteristics of their project against the NSW Health Guidelines to determine if any of the ethical risks described within are present in their project.
If ethical risks are identified, review by an ethics review body should be sought. Please follow the instructions in the QA form regarding submission to the Research Office. If ethical risks are not identified, QA practitioners are free to commence their projects, after adhering to any institutional requirements. NSW Health policy outlines ethics review and approval is not required merely for the mechanism of obtaining publication in a journal [see Q16 in GL 2007_020].
For more information on conducting QA initiatives, please visit the SESLHD Quality Improvement Portal.
Guidance for Clinical Case Report Submission
In keeping with the requirements of some journals, the SESLHD HREC supports a process by which case report proposals are reviewed by an ethical sub-committee. The committee requests that the below form is completed and submitted to SESLHD-RSO@health.nsw.gov.au (subject title: Case Report Submission). A blank copy of the consent form to be used to gain consent from the clinical case must also be submitted for review. Some journals require the use of a specific consent form. A template has also been provided below:
The committee will review your case report for ethical risks and if none are identified will provide you with correspondence confirming that full ethical review is not required. This correspondence may be provided to the editors of Journals requesting clearance from an Ethical Review Committee but is not a statement of ethical approval.